Highlights
-
A low-calorie, nutraceutical dark chocolate fortified with herbal extracts was formulated.
-
Extracts of Crocus sativus L., Rosa damascena, Melissa officinalis L., and Echium amoenum were used.
-
Complex coacervation-spray drying approaches were utilized for the double encapsulation of the extracts.
-
The textural and sensory properties of the designed chocolate were enhanced.
-
The visual characteristics of the samples, in terms of brightness and Chroma, were improved.
- Incubator Center of Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran
Received 30 October 2023, Revised 9 December 2023, Accepted 2 April 2024, Available online 6 April 2024, Version of Record 9 April 2024.
Abstract
Keywords
Introduction
2. Materials and methods
2.1. Preparation of medicinal herb extracts
2.1.1. Preparation of saffron extract
2.1.2. Preparation of rosa extract
2.1.3. Preparation of borage extract
2.1.4. Preparation of balm extract
2.2. Determination of indicator compounds in herbal extracts
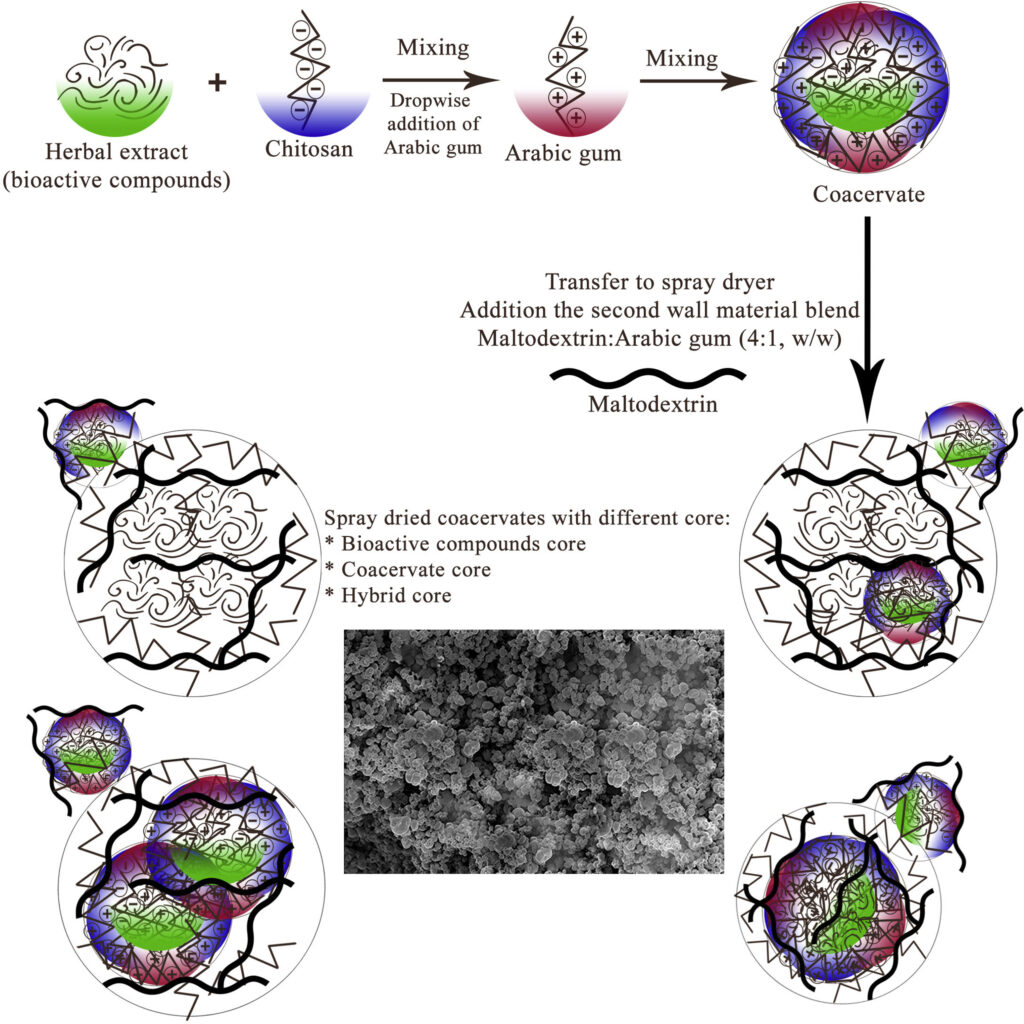
2.3. Encapsulation of herbal extracts
2.3.1. Encapsulation via complex coacervation
2.3.2. Encapsulation via spray drying
2.3.3. Characterization of microcapsules
2.3.3.1. Characterization of complex coacervates
2.3.3.2. Characterization of spray dried coacervates
2.4. Nutraceutical dark chocolate designing
Table 1. The amount of herbal extract microcapsules in different NDCh samples.
| Chocolate sample code | Microencapsulated herbal extract | Total (mg) | |||
|---|---|---|---|---|---|
| Saffron (%) | Borage (%) | Balm (%) | Rosa (%) | ||
| Control | – | – | – | – | 0 |
| F1 | 6 | 75 | 16 | 3 | 500 |
| F2 | 4.5 | 56.5 | 33.9 | 5.1 | 885 |
| F3 | 5.4 | 51.4 | 41 | 2.2 | 730 |
| F4 | 4.6 | 76.3 | 12.3 | 6.8 | 655 |
| F5 | 4 | 50 | 40 | 6 | 750 |
| F6 | 7.4 | 69.4 | 14.8 | 8.4 | 540 |
| F7 | 3.6 | 59.1 | 35.5 | 1.8 | 845 |
| F8 | 6.3 | 78.7 | 12.6 | 2.4 | 635 |
2.5. Nutraceutical dark chocolate characterization
2.5.1. Color indices
2.5.2. Melting properties
2.5.3. Analysis of rheological and textural properties
2.5.4. Assessment the stability of encapsulated bioactive compounds in chocolate sample
2.5.5. Sensory analysis
Table 2. Description of the sensory attributes.
| Sensory attributes | description | |
|---|---|---|
| Appearance | Brightness | The perceived color of an object signifies the correlation between the light it reflects and the light it absorbs. |
| Color | Intensity of brown color | |
| Texture | Hardness | The force that is necessary to cleave the chocolate in two using one’s front teeth. |
| Grittiness | The presence of fine or large particles in the molten chocolate | |
| Melting | The resistance of chocolate to being completely liquid | |
| Taste and aroma | Herbal | Intensity of medicinal herbs extract taste |
| Astringency | Intensity of causing tango to contract. | |
2.6. Statistical analysis
3. Results and discussion
3.1. Complex coacervates characterization
3.1.1. Particle size and particle size distribution
Table 3. Characterization of complex coacervates and spray dried complex coacervates of different herbal extracts.
| Herbal extract | Complex coacervates properties | Spray dried complex coacervates properties | ||||
|---|---|---|---|---|---|---|
| Mean particle size (μm) | PDI | MC (%) | MY (%) | First EE (%) | Second EE (%) | |
| Saffron | 2.1 ± 0.1 a | 0.111 ± 0.02 a | 4.49 ± 0.33 a | 92.1 ± 1.5 a | 90.6 ± 1.7 a | 99.2 ± 0.4 a |
| Borage | 2.2 ± 0.1 a | 0.105 ± 0.01 a | 4.55 ± 0.18 a | 91.9 ± 2.1 a | 90.2 ± 1.9 a | 99.7 ± 1.2 a |
| Balm | 2.1 ± 0.1 a | 0.103 ± 0.03 a | 4.46 ± 0.47 a | 92.5 ± 1.9 a | 91.0 ± 2.2 a | 99.1 ± 1.5 a |
| rosa | 2.1 ± 0.1 a | 0.114 ± 0.01 a | 4.59 ± 0.63 a | 91.6 ± 3.2 a | 91.1 ± 1.8 a | 99.5 ± 0.8 a |
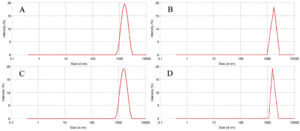
Fig. 1. Particle size distribution of complex coacervates of herbal extracts. (A) Borage, (B) Saffron, (C) Balm, (D) rosa.
3.1.2. Morphology of coacervates
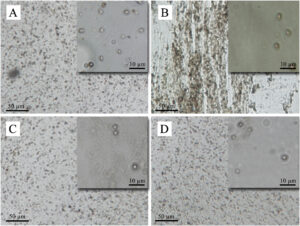
Fig. 2. Optical microscopy images of complex coacervates of herbal extracts. (A) Borage, (B) Saffron, (C) Balm, (D) rosa.
3.2. Spray dried complex coacervates characterization
3.2.1. Moisture content
3.2.2. Microcapsule yield and encapsulation efficiency
3.2.3. Morphological properties of spray dried coacervates
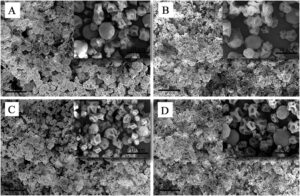
Fig. 3. SEM images of spray dried complex coacervates of herbal extracts. (A) Borage, (B) Saffron, (C) Balm, (D) rosa.
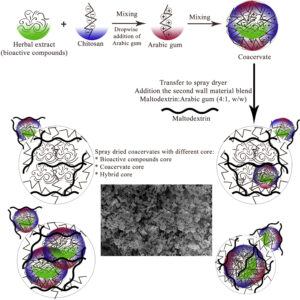
-
Fig. 4. Schematic representation of preparation of herbal extract microcapsules.
Moreover, the compact-interconnected appearance of microcapsules in Fig. 3 could be due to interactions between those CH molecules that did not participate in the complexation process with AG that was added along with MD as the second wall material system. Accordingly, the amine groups of CH could interact with the carboxyl and hydroxyl groups of AG, leading to the formation of a dense-interconnected network.3.3. Nutraceutical dark chocolates characterization
3.3.1. Color indices
The color indices of chocolate are influenced by several factors, including the production process, recipe, and the crystal structure of fat (Saputro et al., 2017). The changes in the color of the NDCh samples were analyzed 24 h after production by computing different color indices (Table 4). The incorporation of microencapsulated herbal extract powders into the samples significantly altered the color profiles of the samples (p < 0.05). The level of lightness (L*) increased in correlation with the amount of spray-dried powders added. The lowest (26.15) and highest (32.11) L* values were recorded for the control and F2 samples (which contained the highest amount of microcapsules), respectively (p > 0.05). An increase in L* value or sample brightness enhances its visual quality. Therefore, it can be inferred that the incorporation of spray-dried powders, regardless of the type of herbal extract, significantly improved the color of dark chocolate samples, making them brighter. Similar results were reported by Hadnađev et al. (2023) and Agibert and Lannes (2018), who worked on fortifying dark chocolate using different microcapsules. The same trend was observed for a* (increased from 6.76 in the control sample to 9.86 in F2) and b* (increased from 4.09 in the control sample to 6.47 in F2). That is, as the amount of microencapsulated herbal extract in the recipe increased, these values significantly increased (p < 0.05), similar to the trend reported by Toker et al. (2018), who worked on fortifying chocolate with encapsulated phytosterols. Other researchers also reported that the addition of encapsulated bioactive compounds significantly changed the color indices of chocolate samples (Lončarević et al., 2018; Lončarević et al., 2019; Toker et al., 2018; Tolve et al., 2018).Table 4. Color indices of the NDCh samples.
Chocolate sample code L* a* b* h C WI Control 26.15 ± 0.214 e 6.76 ± 0.163 f 4.09 ± 0.096 d 0.70 ± 0.01 c 7.90 ± 0.59 f 74.27 ± 1.21 a F1 28.93 ± 0.221 d 7.64 ± 0.099 e 4.88 ± 0.096 c 0.75 ± 0.05 b 9.06 ± 0.41 e 71.64 ± 0115 b F2 32.11 ± 0.124 a 9.86 ± 0.206 a 6.47 ± 0.092 a 0.79 ± 0.03 b 11.79 ± 0.62 a 68.90 ± 0.40 e F3 30.35 ± 0.154 bc 8.63 ± 0.184 c 6.01 ± 0.107 b 0.86 ± 0.02 a 10.52 ± 0.48 cd 70.43 ± 1.09 cd F4 29.97 ± 0.109 bc 8.21 ± 0.131 d 5.83 ± 0.124 b 0.89 ± 0.06 a 10.07 ± 0.74 d 70.75 ± 1.53 c F5 30.44 ± 0.099 b 8.77 ± 0.235 c 6.11 ± 0.112 b 0.86 ± 0.09 a 10.69 ± 0.45 c 70.37 ± 1.39 cd F6 29.22 ± 0.112 d 7.87 ± 0.228 d 4.95 ± 0.055 c 0.74 ± 0.06 bc 9.29 ± 0.58 e 70.17 ± 0.44 d F7 31.69 ± 0.107 a 9.26 ± 0.058 b 6.35 ± 0.218 a 0.84 ± 0.11 a 11.23 ± 0.74 b 69.23 ± 0.72 e F8 29.81 ± 0.087 c 8.11 ± 0.115 d 5.75 ± 0.111 b 0.88 ± 0.08 a 9.94 ± 0.29 d 70.89 ± 1.48 bc The values provided are the averages derived from three trials, with the standard deviation denoted as ±. In each column, values that are followed by distinct lowercase letters are statistically different from one another, with a significance level of less than 0.05.Three factors can be attributed to these observations:- 1.
All the spray-dried powders had a surface color similar to the corresponding herbal extract, albeit with less intensity. Therefore, their incorporation into the formulation enhanced the color indices, especially lightness.
- 2.
The spray-dried powders had a small particle size and narrow particle size distribution. One of the most important factors influencing chocolate color is particle size.
- 3.
The presence of AG in the chocolate formulation, especially in the recipe containing the maximum amount of microencapsulated powder (F2 and F7), may have altered the surface properties of the samples, which directly affect the visual properties of chocolates.
The phenomenon of fat blooming, which is considered a negative quality attribute, occurs when cocoa butter destabilizes, migrates through the chocolate matrix towards the surface, and recrystallizes, resulting in an off-white color. To track the occurrence and intensity of this phenomenon, the whiteness index (WI) is measured (Hadnađev et al., 2023). The WI of chocolate samples varied from 68.90 (F2) to 74.27 (control sample), indicating that the addition of microencapsulated herbal extract significantly decreased this index (p < 0.05). Despite the lowering effect of encapsulated powders on WI, it’s important to note that the appearance of chocolate samples did not show any signs of fat blooming (Fig. 5). The increase in WI could be attributed to the lighter color of encapsulated powders and their impact on enhancing cocoa butter recrystallization. Similar results were published about fortification of chocolate with microcapsules of fish oil (Hadnađev et al., 2023), microcapsules of peanut oil (Agibert & Lannes, 2018), and microcapsules of chia seed oil (Razavizadeh & Tabrizi, 2021).
- 1.
Fig. 5. Nutraceutical dark chocolate samples.
Chroma (C) shows the degree of saturation or color intensity while hue (h) is an indicator of food luminance color. Both C and h were significantly influenced by the addition of microencapsulated herbal extract powders (p < 0.05), with both indices experiencing an increasing trend in parallel to increasing levels of fortification. Accordingly, the value of C varied from 7.90 (control sample) to 11.79 (F2) while h changed from 0.70 to 0.79 for control and F2 samples respectively. It has been shown that both these indices are matrix-dependent factors, i.e., their values significantly alter by changing particle size, particle size distribution, and product density (Afoakwa et al., 2008). Accordingly, a finer matrix scatters more light. Therefore, it can be concluded that adding microencapsulated powder due to effects on chocolate particle size pattern significantly changed the surface visual properties of samples, resulting in a lighter and more saturated appearance than control or those that had lower levels of fortification. These findings are in accordance with those reported by Toker et al. (2018) and Afoakwa et al. (2008).3.3.2. Melting characteristics of chocolate samples
The thermal properties of chocolate are vitally important for product quality and consumer preferences. Accordingly, four essential thermal indices of NDCh samples were quantified using the DSC method (Table 5). Tonset, Tpeak, and Tend respectively varied in the range of 26.39–28.05 °C, 32.23–33.81 °C, and 34.19–35.28 °C, while enthalpy ranged from 28.19 (F3) to 29.03 (F7), with no significant changes (p > 0.05). Incorporation of microencapsulated powders into the chocolate formulation at the highest concentrations (845 and 885 mg/33 g chocolate) resulted in a significant enhancement of Tonset, Tpeak, and Tend. However, at lower levels of fortification (500, 540, 635, 655, 730, and 750 mg/33 g chocolate), these indices did not experience a pivotal change when compared with the control sample (p > 0.05). These observations could be attributed to the presence of AG, especially at higher levels of fortification, which resulted in increasing interactions between biopolymers and the formation of a more stable network. Furthermore, it was shown that by incorporating additional compounds into the chocolate recipe, its melting behavior was altered due to the eutectic effect (Zhang et al., 2020). These results were in accordance with findings by Hadnađev et al. (2023) and Toker et al. (2018), who respectively claimed that incorporating microencapsulated fish oil and eicosapentaenoic and docosahexaenoic acids into dark chocolate significantly changed thermal properties. On the other hand, the intensity of changes between these researches and the present study was different. For instance, in the research by Toker et al. (2018), Tend did not change significantly while others did. These differences could be ascribed to variations in the chocolate recipe (type of sweeteners, presence and type of bulking agent), processing conditions (duration and temperature of each step), type of fortificant (physicochemical properties of bioactive compounds), and level of fortification.Table 5. Thermal properties of the NDCh samples.
| Chocolate sample code | Tonset | Tpeak | Tend | ΔH |
|---|---|---|---|---|
| Control | 26.39 ± 0.109 b | 32.23 ± 0.154 b | 34.19 ± 0.115 b | 28.51 ± 0.115 a |
| F1 | 26.69 ± 0.109 b | 32.38 ± 0.154 a | 34.29 ± 0.115 b | 28.34 ± 0.115 a |
| F2 | 28.05 ± 0.109 a | 33.81 ± 0.154 a | 35.28 ± 0.115 a | 28.66 ± 0.115 a |
| F3 | 27.55 ± 0.109 b | 32.82 ± 0.154 b | 34.68 ± 0.115 b | 28.19 ± 0.115 a |
| F4 | 27.13 ± 0.109 b | 32.69 ± 0.154 b | 34.54 ± 0.115 b | 28.75 ± 0.115 a |
| F5 | 27.57 ± 0.109 b | 32.87 ± 0.154 b | 34.73 ± 0.115 b | 29.12 ± 0.115 a |
| F6 | 26.68 ± 0.109 b | 32.35 ± 0.154 b | 34.28 ± 0.115 b | 28.84 ± 0.115 a |
| F7 | 28.12 ± 0.109 a | 33.74 ± 0.154 a | 35.16 ± 0.115 a | 29.03 ± 0.115 a |
| F8 | 27.11 ± 0.109 b | 32.70 ± 0.154 b | 34.51 ± 0.115 b | 28.33 ± 0.115 a |
3.3.3. Rheological and textural characteristics of chocolate samples
The rheological behavior of NDCh samples was assessed by computing Casson’s yield stress and viscosity (Table 6). The viscosity of such a system is governed by particle size distribution, production process, and formulation (Afoakwa et al., 2008). As the former and latter were altered in this study, tracking the changes in rheological behavior is profoundly important as they are directly correlated with texture and consumer preferences. Incorporation of herbal extract microcapsules into the chocolate formulation at high levels of fortification had a significant effect on Casson viscosity (p < 0.05). The Casson viscosity ranged between 2.88 and 2.95 Pa s, respectively for control and F2 samples. A significant enhancement of Casson viscosity in two samples, F2 and F7, which contained 885 and 845 mg of microcapsules per 33 g of chocolate, may be due to the presence of a higher amount of AG than other formulations. AG can absorb water, interact with other biomolecules in the chocolate matrix, and consequently, increase the viscosity. In line with this, Hadnađev et al. (2023), Toker et al. (2018), and Agibert and Lannes (2018) reported that the inclusion of microcapsules into the chocolate recipe resulted in a significant increment of Casson plastic viscosity.Table 6. Rheological and textural characteristics of the NDCh samples.
| Chocolate sample code | Rheological properties | Textural properties | ||
|---|---|---|---|---|
| Casson plastic viscosity (Pas) | Casson yield stress (Pa) | Breaking force (N) | Brittleness (mm) | |
| Control | 2.88 ± 0.028 b | 6.85 ± 0.016 c | 21.55 ± 0.404 c | 69.33 ± 0.209 c |
| F1 | 2.89 ± 0.030 b | 6.88 ± 0.024 c | 21.63 ± 0.519 c | 69.24 ± 0.221 c |
| F2 | 2.95 ± 0.025 a | 7.22 ± 0.206 a | 21.96 ± 0.196 a | 67.21 ± 0.129 a |
| F3 | 2.90 ± 0.036 b | 7.03 ± 0.029 b | 21.69 ± 0.226 b | 67.33 ± 0.154 b |
| F4 | 2.89 ± 0.031 b | 6.90 ± 0.022 c | 21.68 ± 0.617 c | 69.75 ± 0.115 c |
| F5 | 2.91 ± 0.023 b | 6.99 ± 0.016 b | 21.73 ± 1.004 b | 67.17 ± 0.199 b |
| F6 | 2.88 ± 0.032 b | 6.89 ± 0.023 c | 21.70 ± 0.251 c | 69.50 ± 0.212 c |
| F7 | 2.94 ± 0.028 a | 7.15 ± 0.026 a | 21.98 ± 0.471 a | 67.39 ± 0.159 a |
| F8 | 2.90 ± 0.022 b | 6.91 ± 0.018 c | 21.68 ± 1.055 c | 69.7167 ± 0.221 c |
3.3.4. Stability of bioactive compounds in chocolate
The amounts of crocin and TA in the corresponding extract, spray-dried coacervates, and chocolate (F7) are shown in Table 7. Chocolate sample formulation F7 was selected based on the sensory analysis results (Table 8). The amounts of these compounds in the extract and spray-dried coacervates were almost the same (p > 0.05). Following the incorporation of spray-dried coacervates into the chocolate formulation, the amounts of crocin and TA significantly decreased (p < 0.05). Crocin decreased from 282 to 239 (∼15% loss), while the amount of TA decreased from 88.64 to 67.09 mg L−1 (∼24.5% loss). This loss was probably due to the heating during conching process (50 °C-15 min), as crocin and anthocyanin are both heat-sensitive compounds. Moreover, these results showed that the sensitivity of anthocyanin was greater than that of crocin, as it was destroyed 10% more. Despite the fact that about 15% and 24.5% of the bioactive compounds of borage and saffron were destroyed, respectively, it can be said that the chocolate is still an appropriate delivery system.Table 7. The amounts of bioactive compounds in extract, spray dried coacervates, and chocolate sample.
| Medicinal herb | Extract | Spray dried coacervates | Chocolate (F7) | Loss in chocolate (%) |
|---|---|---|---|---|
| Saffron (crocin,A1cm1%(λ440)) | 286.96 ± 1.66 a | 282.43 ± 0.39 a | 239 ± 1.18 b | 16.43 |
| Borage (TA, mg L−1) | 89.30 ± 0.47 a | 88.64 ± 0.14 a | 67.09 ± 0.52 b | 24.87 |
| Balm (TPC, GAE) | 53.22 ± 0.71 a | 52.77 ± 0.57 a | – | – |
| rosa (TPC, GAE) | 241.65 ± 1.23 a | 240.28 ± 2.11 a | – | – |
Table 8. Sensory properties of different NDCh samples.
| Sensory attributes | Control | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | Brightness | 5.89 ± 0.11 c | 6.25 ± 0.25 b | 6.76 ± 0.16 a | 6.41 ± 1.12 b | 6.24 ± 0.52 b | 6.39 ± 0.48 b | 6.25 ± 1.22 b | 6.60 ± 0.77 a | 6.20 ± 0.38 b |
| Color | 6.87 ± 0.36 a | 6.33 ± 0.63 b | 5.84 ± 0.69 c | 6.10 ± 0.58 b | 6.35 ± 0.83 b | 6.13 ± 0.55 b | 6.35 ± 0.49 b | 5.89 ± 0.69 c | 6.30 ± 0.44 b | |
| Texture | Hardness | 6.12 ± 0.59 b | 6.20 ± 0.47 b | 6.45 ± 1.05 a | 6.11 ± 0.55 b | 6.17 ± 0.63 b | 6.25 ± 0.48 b | 6.15 ± 0.26 b | 6.48 ± 0.30 a | 6.28 ± 0.37 b |
| Grittiness | 1.85 ± 0.31 c | 4.10 ± 0.18 b | 5.17 ± 0.43 a | 5.00 ± 0.28 a | 4.45 ± 0.56 b | 5.05 ± 0.29 a | 4.15 ± 0.73 b | 5.15 ± 0.44 a | 4.22 ± 0.19 b | |
| Melting | 2.15 ± 0.49 b | 2.55 ± 0.82 b | 3.88 ± 0.70 a | 2.40 ± 0.35 b | 2.67 ± 0.49 b | 2.39 ± 0.51 b | 2.71 ± 0.22 b | 3.95 ± 0.35 a | 2.25 ± 0.88 b | |
| Taste and aroma | Herbal | 1.21 ± 0.21 c | 2.59 ± 0.39 b | 3.62 ± 0.17 a | 3.56 ± 0.93 a | 2.16 ± 0.78 b | 3.74 ± 0.89 a | 2.38 ± 0.18 b | 3.66 ± 0.49 a | 2.30 ± 0.65 b |
| Astringency | 1.12 ± 0.44 c | 2.64 ± 0.76 b | 3.87 ± 0.26 a | 3.88 ± 0.73 a | 2.45 ± 0.66 b | 3.79 ± 0.17 a | 2.54 ± 0.59 b | 3.90 ± 0.83 a | 2.21 ± 0.70 b | |
3.3.5. Sensory analysis of chocolate samples
The sensory attributes of NDCh samples were analyzed and the results are presented in Table 8. The panelists were able to perceive the presence of microencapsulated herbal extract at high levels of fortification. Accordingly, the samples containing 885 and 845 mg per 33 g of chocolate were found to have the highest brightness intensity (p < 0.05). The intensity of the brown color was also significantly affected by the fortification process, with the control sample having the highest intensity (p < 0.05), which aligns with the color analysis results (Table 4). The intensity of sandiness or grittiness in NDCh samples significantly increased with the amount of microcapsules, consistent with findings reported by Lončarević et al. (2019) who worked on fortifying white chocolate with green tea extract microcapsules. Similarly, as the amount of microcapsules increased, so did the herbal intensity. Finally, the astringency intensity in NDCh sampels was significantly higher than that of the control sample, especially in samples with the highest amount of balm extract. This is in line with Sik et al. (2021), who reported that the astringency of dark chocolate fortified with balm extract was significantly higher than that of the control sample. It can be concluded from Table 7 that the samples fortified with minimal amounts of balm and borage microcapsules, and maximal levels of saffron and rosa, received higher scores than even the control sample.4. Conclusion
A dark chocolate was fortified by adding extracts of four medicinal herbs, which were double encapsulated using CH, AG, and MD. The fortified chocolate exhibited significantly higher hardness, brightness, and melting temperature, especially at the highest levels of fortification. The Casson’s viscosity and yield stress of the chocolate samples increased in parallel with the increase in AG concentration, which was attributed to the interaction of functional groups of gum with those of the other ingredients. The snap of the chocolate was improved by the addition of microcapsules, while some sensory attributes such as astringency were negatively influenced at the highest levels of concentration. This study presents an effective strategy for safeguarding the bioactive compounds found in medicinal herbs, while also offering a worthwhile delivery system for these health-enhancing compounds. The findings have the potential to significantly improve the mental and physical well-being of diverse communities by paving the way for the creation of a range of functional foods enriched with these protected bioactive compounds.CRediT authorship contribution statement
Hamid Rajabi: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft. Samineh Sedaghati: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing.Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgement This work was financially supported by the Ario Rad Mehr Torshiz Co.- Mirmohannay (Project number: RD2023-ICGAU-14077).Data availability
Data will be made available on request.References
-
- Abedini et al., 2023A. Abedini, S. Dakhili, S. Bazzaz, S. Kamaladdin Moghaddam, M. Mahmoudzadeh, H. AndishmandFortification of chocolates with high‐value‐added plant‐based substances: Recent trends, current challenges, and future prospectsFood Science and Nutrition (2023)Google Scholar
-
- Afoakwa et al., 2008E.O. Afoakwa, A. Paterson, M. Fowler, J. VieiraParticle size distribution and compositional effects on textural properties and appearance of dark chocolatesJournal of Food Engineering, 87 (2) (2008), pp. 181-190View PDFView articleView in ScopusGoogle Scholar
-
- Agibert and Lannes, 2018S.A. Agibert, S.C.d.S. LannesDark chocolate with a high oleic peanut oil microcapsule contentJournal of the Science of Food and Agriculture, 98 (15) (2018), pp. 5591-5597CrossrefView in ScopusGoogle Scholar
-
- Ahmad et al., 2022S. Ahmad, A. Azhar, P. Tikmani, H. Rafique, A. Khan, H. Mesiya, H. SaeedA randomized clinical trial to test efficacy of chamomile and saffron for neuroprotective and anti-inflammatory responses in depressive patientsHeliyon, 8 (10) (2022), Article e10774View PDFView articleView in ScopusGoogle Scholar
-
- Beckett, 2019S.T. BeckettThe science of chocolateRoyal Society of Chemistry (2019)Google Scholar
-
- Bordón et al., 2021M.G. Bordón, A.J. Paredes, N.M. Camacho, M.C. Penci, A. González, S.D. Palma, …, M.L. MartinezFormulation, spray-drying and physicochemical characterization of functional powders loaded with chia seed oil and prepared by complex coacervationPowder Technology, 391 (2021), pp. 479-493View PDFView articleView in ScopusGoogle Scholar
-
- Carlan et al., 2020I.C. Carlan, B.N. Estevinho, F. RochaProduction of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materialsCanadian Journal of Chemical Engineering, 98 (8) (2020), pp. 1682-1695View at publisherCrossrefView in ScopusGoogle Scholar
-
- Cimini et al., 2013A. Cimini, R. Gentile, B. D’Angelo, E. Benedetti, L. Cristiano, M.L. Avantaggiati, …, G. DesideriCocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathwayJournal of Cellular Biochemistry, 114 (10) (2013), pp. 2209-2220View at publisherCrossrefView in ScopusGoogle Scholar
-
- Couto et al., 2013R.O. Couto, F.S. Martins, L.T. Chaul, E.C. Conceição, L.A.P. Freitas, M.T.F. Bara, J.R. PaulaSpray drying of eugenia dysenterica extract: Effects of in-process parameters on product qualityRevista brasileira de Farmacognosia, 23 (1) (2013), pp. 115-123View PDFView articleView in ScopusGoogle Scholar
-
- Dean et al., 2016L. Dean, C. Klevorn, B. HessMinimizing the negative flavor attributes and evaluating consumer acceptance of chocolate fortified with peanut skin extractsJournal of Food Science, 81 (11) (2016), pp. S2824-S2830View in ScopusGoogle Scholar
-
- Engler et al., 2004M.B. Engler, M.M. Engler, C.Y. Chen, M.J. Malloy, A. Browne, E.Y. Chiu, …, J. BlumbergFlavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adultsJournal of the American College of Nutrition, 23 (3) (2004), pp. 197-204View at publisherCrossrefView in ScopusGoogle Scholar
-
- Farajdokht et al., 2020F. Farajdokht, A. Vosoughi, M. Ziaee, M. Araj-Khodaei, J. Mahmoudi, S. Sadigh-EteghadThe role of hippocampal GABAA receptors on anxiolytic effects of Echium amoenum extract in a mice model of restraint stressMolecular Biology Reports, 47 (9) (2020), pp. 6487-6496CrossrefView in ScopusGoogle Scholar
-
- Ferreira and Nicoletti, 2021S. Ferreira, V.R. NicolettiMicroencapsulation of ginger oil by complex coacervation using atomization: Effects of polymer ratio and wall material concentrationJournal of Food Engineering, 291 (2021), Article 110214View PDFView articleView in ScopusGoogle Scholar
-
- Glicerina et al., 2016V. Glicerina, F. Balestra, M. Dalla Rosa, S. RomaniMicrostructural and rheological characteristics of dark, milk and white chocolate: A comparative studyJournal of Food Engineering, 169 (2016), pp. 165-171View PDFView articleView in ScopusGoogle Scholar
-
- Gültekin-Özgüven et al., 2016M. Gültekin-Özgüven, A. Karadağ, Ş. Duman, B. Özkal, B. ÖzçelikFortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studiesFood Chemistry, 201 (2016), pp. 205-212View PDFView articleView in ScopusGoogle Scholar
-
- Hadnađev et al., 2023M. Hadnađev, M. Kalić, V. Krstonošić, N. Jovanović-Lješković, T. Erceg, D. Škrobot, T. Dapčević-HadnađevFortification of chocolate with microencapsulated fish oil: Effect of protein wall material on physicochemical properties of microcapsules and chocolate matrixFood Chemistry X, 17 (2023), Article 100583View PDFView articleView in ScopusGoogle Scholar
-
- Haybar et al., 2018H. Haybar, A.Z. Javid, M.H. Haghighizadeh, E. Valizadeh, S.M. Mohaghegh, A. MohammadzadehThe effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable anginaClinical nutrition ESPEN, 26 (2018), pp. 47-52View PDFView articleView in ScopusGoogle Scholar
-
- Hernández-Nava et al., 2020R. Hernández-Nava, A. López-Malo, E. Palou, N. Ramírez-Corona, M.T. Jiménez-MunguíaEncapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray dryingFood Hydrocolloids, 109 (2020), Article 106077View PDFView articleView in ScopusGoogle Scholar
-
- Jackson et al., 2019S.E. Jackson, L. Smith, J. Firth, I. Grabovac, P. Soysal, A. Koyanagi, …, N. VeroneseIs there a relationship between chocolate consumption and symptoms of depression? A cross‐sectional survey of 13,626 us adultsDepression and Anxiety, 36 (10) (2019), pp. 987-995CrossrefView in ScopusGoogle Scholar
-
- Jafari et al., 2017S.M. Jafari, I. Katouzian, H. Rajabi, M. GanjeBioavailability and release of bioactive components from nanocapsulesNanoencapsulation technologies for the food and nutraceutical industries, Elsevier (2017), pp. 494-523View PDFView articleView in ScopusGoogle Scholar
-
- Jafari et al., 2020S.-M. Jafari, M.Z. Tsimidou, H. Rajabi, A. KyriakoudiBioactive ingredients of saffron: Extraction, analysis, applicationsSaffron, Elsevier (2020), pp. 261-290View PDFView articleView in ScopusGoogle Scholar
-
- Lee, Durst, & Wrolstad, 2005J. Lee, R.W. Durst, R.E. WrolstadDetermination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative studyJournal of AOAC International, 88 (5) (2005), pp. 1269-1278CrossrefView in ScopusGoogle Scholar
-
- Lončarević et al., 2018I. Lončarević, B. Pajin, A. Fišteš, V.T. Šaponjac, J. Petrović, P. Jovanović, …, D. ZarićEnrichment of white chocolate with blackberry juice encapsulate: Impact on physical properties, sensory characteristics and polyphenol contentLwt, 92 (2018), pp. 458-464View PDFView articleView in ScopusGoogle Scholar
-
- Lončarević et al., 2019I. Lončarević, B. Pajin, V. Tumbas Šaponjac, J. Petrović, J. Vulić, A. Fišteš, P. JovanovićPhysical, sensorial and bioactive characteristics of white chocolate with encapsulated green tea extractJournal of the Science of Food and Agriculture, 99 (13) (2019), pp. 5834-5841CrossrefView in ScopusGoogle Scholar
-
- Lv et al., 2014Y. Lv, F. Yang, X. Li, X. Zhang, S. AbbasFormation of heat-resistant nanocapsules of jasmine essential oil via gelatin/gum Arabic based complex coacervationFood Hydrocolloids, 35 (2014), pp. 305-314View PDFView articleView in ScopusGoogle Scholar
-
- Marsanasco et al., 2016M. Marsanasco, V. Calabró, B. Piotrkowski, N.S. Chiaramoni, V. del, S. AlonsoFortification of chocolate milk with omega‐3, omega‐6, and vitamins E and C by using liposomesEuropean Journal of Lipid Science and Technology, 118 (9) (2016), pp. 1271-1281CrossrefView in ScopusGoogle Scholar
-
- Martin and Ramos, 2021M.Á. Martin, S. RamosImpact of cocoa flavanols on human healthFood and Chemical Toxicology, 151 (2021), Article 112121View PDFView articleView in ScopusGoogle Scholar
-
- Martin et al., 2009F.-P.J. Martin, S. Rezzi, E. Peré-Trepat, B. Kamlage, S. Collino, E. Leibold, …, S. KochharMetabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjectsJournal of Proteome Research, 8 (12) (2009), pp. 5568-5579CrossrefView in ScopusGoogle Scholar
-
- Muhammad et al., 2018D.R.A. Muhammad, A.D. Saputro, H. Rottiers, D. Van de Walle, K. DewettinckPhysicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticlesEuropean Food Research and Technology, 244 (2018), pp. 1185-1202CrossrefView in ScopusGoogle Scholar
-
- Mursu et al., 2004J. Mursu, S. Voutilainen, T. Nurmi, T.H. Rissanen, J.K. Virtanen, J. Kaikkonen, …, J.T. SalonenDark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humansFree Radical Biology and Medicine, 37 (9) (2004), pp. 1351-1359View PDFView articleView in ScopusGoogle Scholar
-
- Rajabi et al., 2015H. Rajabi, M. Ghorbani, S.M. Jafari, A.S. Mahoonak, G. RajabzadehRetention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materialsFood Hydrocolloids, 51 (2015), pp. 327-337View PDFView articleView in ScopusGoogle Scholar
-
- Rajabi et al., 2021H. Rajabi, S.M. Jafari, J. Feizi, M. Ghorbani, S.A. MohajeriSurface-decorated graphene oxide sheets with nanoparticles of chitosan-Arabic gum for the separation of bioactive compounds: A case study for adsorption of crocin from saffron extractInternational Journal of Biological Macromolecules, 186 (2021), pp. 1-12View PDFView articleView in ScopusGoogle Scholar
-
- Rajabi et al., 2020H. Rajabi, S.M. Jafari, J. Feizy, M. Ghorbani, S.A. MohajeriPreparation and characterization of 3D graphene oxide nanostructures embedded with nanocomplexes of chitosan-gum Arabic biopolymersInternational Journal of Biological Macromolecules, 162 (2020), pp. 163-174View PDFView articleView in ScopusGoogle Scholar
-
- Rajabi et al., 2019H. Rajabi, S.M. Jafari, G. Rajabzadeh, M. Sarfarazi, S. SedaghatiChitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive componentsColloids and Surfaces A: Physicochemical and Engineering Aspects, 578 (2019), Article 123644View PDFView articleView in ScopusGoogle Scholar
-
- Rajabi, Sedaghati, Rajabzadeh, & Sani, 2024H. Rajabi, S. Sedaghati, G. Rajabzadeh, A.M. SaniCharacterization of microencapsulated spinach extract obtained by spray-drying and freeze-drying techniques and its use as a source of chlorophyll in a chewing gum based on Pistacia atlanticaFood Hydrocolloids, 150 (2024), p. 109665View PDFView articleView in ScopusGoogle Scholar
-
- Rasooli et al., 2021T. Rasooli, M. Nasiri, Z. Kargarzadeh Aliabadi, M.R. Rajabi, S. Feizi, M. Torkaman, …, M. AbbasiRosa damascena mill for treating adults’ anxiety, depression, and stress: A systematic review and dose–response meta‐analysis of randomized controlled trialsPhytotherapy Research, 35 (12) (2021), pp. 6585-6606CrossrefView in ScopusGoogle Scholar
-
- Ravichandran et al., 2014K. Ravichandran, R. Palaniraj, N.M.M.T. Saw, A.M. Gabr, A.R. Ahmed, D. Knorr, I. SmetanskaEffects of different encapsulation agents and drying process on stability of betalains extractJournal of Food Science and Technology, 51 (2014), pp. 2216-2221CrossrefView in ScopusGoogle Scholar
-
- Razavizadeh and Tabrizi, 2021B.M. Razavizadeh, P. TabriziCharacterization of fortified compound milk chocolate with microcapsulated chia seed oilLwt, 150 (2021), Article 111993View PDFView articleView in ScopusGoogle Scholar
-
- Ribeiro et al., 2020A.M. Ribeiro, M. Shahgol, B.N. Estevinho, F. RochaMicroencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum Arabic, starch and maltodextrinFood Hydrocolloids, 108 (2020), Article 106029View PDFView articleView in ScopusGoogle Scholar
-
- Rojas-Moreno et al., 2018S. Rojas-Moreno, F. Cárdenas-Bailón, G. Osorio-Revilla, T. Gallardo-Velázquez, J. Proal-NájeraEffects of complex coacervation-spray drying and conventional spray drying on the quality of microencapsulated orange essential oilJournal of Food Measurement and Characterization, 12 (2018), pp. 650-660View in ScopusGoogle Scholar
-
- Samanta et al., 2022S. Samanta, T. Sarkar, R. Chakraborty, M. Rebezov, M.A. Shariati, M. Thiruvengadam, K.R. RengasamyDark chocolate: An overview of its biological activity, processing, and fortificationapproachesCurrent Research in Food Science (2022)Google Scholar
-
- Santos et al., 2014M.G. Santos, D.A. Carpinteiro, M. Thomazini, G.A. Rocha-Selmi, A.G. da Cruz, C.E. Rodrigues, C.S. Favaro-TrindadeCoencapsulation of xylitol and menthol by double emulsion followed by complex coacervation and microcapsule application in chewing gumFood Research International, 66 (2014), pp. 454-462View PDFView articleView in ScopusGoogle Scholar
-
- Saputro et al., 2017A.D. Saputro, D. Van de Walle, S. Kadivar, M.D. Bin Sintang, P. Van der Meeren, K. DewettinckInvestigating the rheological, microstructural and textural properties of chocolates sweetened with palm sap-based sugar by partial replacementEuropean Food Research and Technology, 243 (2017), pp. 1729-1738CrossrefView in ScopusGoogle Scholar
-
- Sarfarazi and Mohebbi, 2020M. Sarfarazi, M. MohebbiAn investigation into the crystalline structure, and the rheological, thermal, textural and sensory properties of sugar-free milk chocolate: Effect of inulin and maltodextrinJournal of Food Measurement and Characterization, 14 (2020), pp. 1568-1581CrossrefView in ScopusGoogle Scholar
-
- Sayyah et al., 2006M. Sayyah, M. Sayyah, M. KamalinejadA preliminary randomized double blind clinical trial on the efficacy of aqueous extract of Echium amoenum in the treatment of mild to moderate major depressionProgress in Neuro-Psychopharmacology and Biological Psychiatry, 30 (1) (2006), pp. 166-169View PDFView articleView in ScopusGoogle Scholar
-
- Schröder et al., 2023P. Schröder, S. Cord-Landwehr, M. Schönhoff, C. CramerComposition and charge compensation in chitosan/gum Arabic complex coacervates in dependence on pH and salt concentrationBiomacromolecules, 24 (3) (2023), pp. 1194-1208View articleCrossrefView in ScopusGoogle Scholar
-
- Shafiee et al., 2018M. Shafiee, S. Arekhi, A. Omranzadeh, A. SahebkarSaffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of actionJournal of Affective Disorders, 227 (2018), pp. 330-337View PDFView articleView in ScopusGoogle Scholar
-
- Shiina et al., 2009Y. Shiina, N. Funabashi, K. Lee, T. Murayama, K. Nakamura, Y. Wakatsuki, …, I. KomuroAcute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adultsInternational Journal of Cardiology, 131 (3) (2009), pp. 424-429View PDFView articleView in ScopusGoogle Scholar
-
- Sik et al., 2021B. Sik, E.H. Lakatos, V. Kapcsandi, R. Szekelyhidi, Z. AjtonyExploring the rosmarinic acid profile of dark chocolate fortified with freeze-dried lemon balm extract using conventional and non-conventional extraction techniquesLwt, 147 (2021), Article 111520View PDFView articleView in ScopusGoogle Scholar
-
- Singleton et al., 1999V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventós[14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagentMethods in Enzymology, 299 (1999), pp. 152-178ElsevierView PDFView articleGoogle Scholar
-
- Sukri et al., 2020N. Sukri, R.R. Multisona, Zaida, R.A. Saputra, Mahani, B. NurhadiEffect of maltodextrin and Arabic gum ratio on physicochemical characteristic of spray dried propolis microcapsulesInternational Journal of Food Engineering, 17 (2) (2020), pp. 159-165Google Scholar
-
- Toker et al., 2018O.S. Toker, N. Konar, I. Palabiyik, H.R. Pirouzian, S. Oba, D.G. Polat, …, O. SagdicFormulation of dark chocolate as a carrier to deliver eicosapentaenoic and docosahexaenoic acids: Effects on product qualityFood Chemistry, 254 (2018), pp. 224-231View PDFView articleView in ScopusGoogle Scholar
-
- Tolve et al., 2018R. Tolve, N. Condelli, M.C. Caruso, D. Barletta, F. Favati, F. GalganoFortification of dark chocolate with microencapsulated phytosterols: Chemical and sensory evaluationFood & Function, 9 (2) (2018), pp. 1265-1273CrossrefView in ScopusGoogle Scholar
-
- Zhang et al., 2020Z. Zhang, J. Song, W.J. Lee, X. Xie, Y. WangCharacterization of enzymatically interesterified palm oil-based fats and its potential application as cocoa butter substituteFood Chemistry, 318 (2020), Article 126518View PDFView articleView in ScopusGoogle Scholar